Cyanobacteria, or blue-green algae, is the organism that primarily produces microcystin in a Harmful Algal Bloom (HABs). The reason cyanobacteria produce toxins is still being studied, but nevertheless, is a problem that requires careful consideration to manage and understand.
The relevance of phycocyanin and chlorophyll-a in LG Sonic monitoring
Phycocyanin and chlorophyll-a are pigments blue-green algae and green algae produce. These pigments are measured by LG Sonic’s MPC-Buoys to gauge the presence of algae and help to draw inferences to determine if the algal population is responding to the ultrasonic frequencies being used. If phycocyanin and chlorophyll-a increase, that tells LG Sonic’s project managers and scientists that a different ultrasonic frequency is needed to manage the algal population.
Figure 1: LG Sonic’s ultrasound algae control device, MPC Buoy.
Since cyanobacteria have a short life cycle, this type of algae can adapt to ultrasound over time. Each algal population contains unique and ever-changing genetics with each new generation. This causes variation in their resistance to ultrasound and their ability to produce toxins. With a short lifespan, these variations and changes can happen rapidly.
LG Sonic combats ultrasound resistance by remotely changing ultrasonic programs and offering Interactive Algae Control (IAC) services as the data indicates an adaptation. Currently, there are no known technologies that can sample microcystin autonomously, so phycocyanin and chlorophyll-a are used to evaluate the likelihood of a bloom that could produce toxins.
Microcystin production in cyanobacteria
As mentioned previously, cyanobacterial populations have varying genetics that may allow the production of toxins. Sometimes the gene to produce toxins is present in the algal community, and sometimes it isn’t. Even if these genetics are present in an algal population, it is difficult to know if toxins will be produced. Many scientific papers state that environmental conditions such as pH, temperature, and nutrients may play a role in the expression of these toxin-producing genes [1].
There are other studies that have also shown that high Microcystis density (the genus of cyanobacteria that is most associated with microcystin production) doesn’t necessarily correlate with high toxin concentrations either. Overall, it can be a little unpredictable. Microcystin is a very stable molecule. The half-life is about 10 weeks [2, 3]. The half-life is the amount of time it takes for half of the molecule to degrade or break down. This means that the toxins produced by a HAB can persist in the environment for up to 20 weeks.
Correlation between microcystin, phycocyanin and chlorophyll-a
Since autonomous means of measuring microcystin are lacking, there is value in understanding if there are measurable parameters that correspond to microcystin concentrations. Recent studies have indicated that microcystin is positively correlated with phycocyanin and chlorophyll-a [4, 5].
This means that if phycocyanin and/or chlorophyll are increasing, there is a likelihood microcystin concentrations are increasing as well. Unfortunately, it’s only a likelihood and based on probability. Therefore, one cannot definitively say that if phycocyanin or chlorophyll-a is at x-value, then microcystin must be at y-value.
This is likely since algal gene expression and toxin production varies, as discussed above. However, phycocyanin and chlorophyll-a can still be seen as important tools in understanding the likelihood of toxin production. According to a study conducted in 2016, there is a 50% chance of exceeding microcystin-LR concentrations of 0.3, 1, 1.6, and 2 μg/L when chlorophyll-a concentrations are 23.4, 67.0, 83.5, and 108.5 μg/L, respectively [4].
Summarized, a chlorophyll-a reading of 67.0 μg/L reveals there is a 50% chance that microcystin-LR will be 1 μg/L. This is shown visually in Figure 2, below.
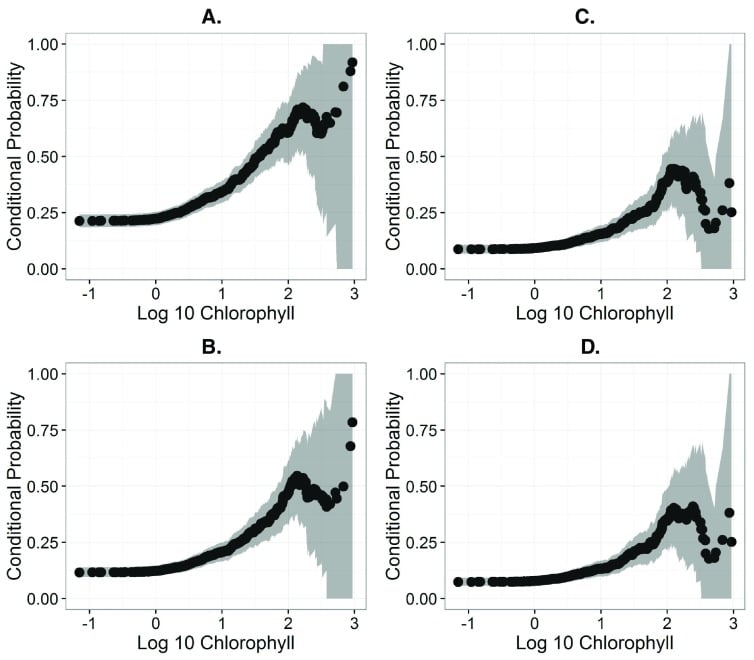
Figure 2: Conditional probability plots showing association between the probability of exceeding various microcystin-LR (MLR) health advisory levels. A. Plot for US EPA child (0.3 μg/L). B. Plot for WHO drinking (1 μg/L). C. Plot for US EPA adult (1.6 μg/L). D. Plot for WHO recreational (2 μg/L) [4].
Similar conclusions have been drawn in modern scientific research for phycocyanin. As discussed, phycocyanin is the primary pigment of blue-green algae or cyanobacteria in freshwater systems. According to a study conducted in 2024 and as shown in Figure 3 below, phycocyanin was found to be significantly and positively correlated to microcystin concentrations with 95% confidence [5].
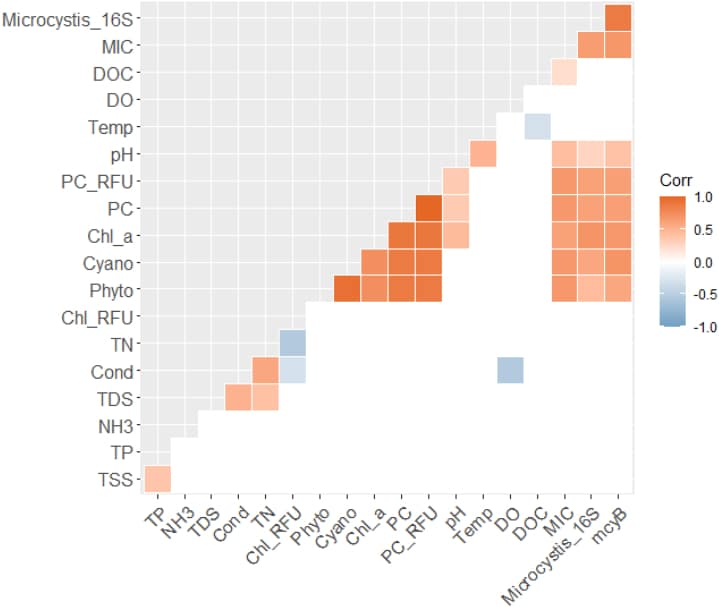
Figure 3: Correlogram showing Spearman’s correlation results among 5 drinking water sources. Only parameters showing significant correlations (p < 0.05) were highlighted. Conductivity (Cond), dissolved oxygen (DO), pH, temperature (Temp), total suspended solids (TSS), total dissolved solids (TDS), dissolved organic carbon (DOC), ammonia (NH3), total nitrogen (TN), total phosphorus (TP), fluorescence of phycocyanin (PC_RFU), fluorescence of chlorophyll (Chl_RFU), extracted chlorophyll-a (Chl_a), phytoplankton cell density (Phyto), cyanobacterial cell density (Cyano), microcystins (MIC), Microcystis 16S rRNA gene (Microcystis_16S), and microcystin-producing mcyB gene (mcyB). [5]
However, the authors indicate more research is required to determine how environmental conditions, algal behavior, and depth influence cyanobacteria’s production of phycocyanin. In short, it can be more difficult to put a direct value-per-value comparison between microcystin and phycocyanin, regardless of the strong correlation. While the research in this brief literature review did not provide a numerical side-by-side comparison for microcystin and phycocyanin, it shows that phycocyanin is an important tool in assessing the growth of cyanobacteria and the possible production of microcystin.
In summary, varying genetic factors and environmental conditions that influence whether cyanobacteria produce toxins in the first place can make direct comparisons complicated. Still, phycocyanin and chlorophyll-a are good data points to measure and compare, especially when the presence of toxins is known. This helps us understand growth and change in the blue-green algae population that are most likely responsible for microcystin production.
References
1. Rinta-Kanto, J., Konopko, E., DeBruyn, J., Bourbonniere, R., Boyer, G., Wilhelm, S., (2009). Lake Erie Microcystis: Relationship between microcystin production, dynamics of genotypes and environmental parameters in a large lake. Harmful Algae. 8(5): 665-673.
2. Butler, N. & Carlisle, J.C. & Linville, Regina & Washburn, Barbara. (2009). Microcystins: a brief overview of their toxicity and effects, with special reference to fish, wildlife, and livestock. OEHHA Ecotoxicology. 1-2.
3. Tsuji K., Watanuki T., Kondo F., Watanabe MF., Suzuki S., Nakazawa H., Suzuki M., Uchida H., Harada KI. (1995). Stability of microcystins from cyanobacteria–II. Effect of UV light on decomposition and isomerization. Toxicon. 33(12): 1619-31.
4. Hollister, J., Kreakie, B. (2016). Associations between chlorophyll a and various microcystin-LR health advisory concentrations. F1000Research. 5. 10.12688/f1000research.7955.1.
5. Hsu, T.D., Caraballo, Y.A., Wu, M. (2024). An investigation of cyanobacteria, cyanotoxins and environmental variables in selected drinking water treatment plants in New Jersey. Heliyon, 10(11).
